Today, there are more than 10,000 types of medical devices available. The selection of appropriate medical equipment always depends on local, regional or national requirements; factors to consider include the type of health facility where the devices are to be used, the health work force available and the burden of disease experienced in the specific catchment area. It is therefore impossible to make a list of core medical equipment which would be exhaustive and/or universally applicable.
Patients in health care settings receive food, medication and other therapies through a variety devices, or delivery systems, such as syringes, catheters, and tubing sets that connect to each other. Connectors are the parts of devices that attach tubing, catheters and syringes to other medical devices.
Medical devices are often packaged together in tubing sets or co-packaged with another device (e.g. feeding set and enteral feeding tube). These sets comprise all the parts needed to use the tubing for its intended purpose, including the connectors that attach tubes to the other parts of the set or to other devices.
In a typical hospital setting, several different types of medical devices, each with their own connections, may be in use at the same time on a single patient. In specialized settings, such as intensive care units (ICUs), cardiac care units, or emergency departments, patients may require dozens of different devices at once.
Devices that need to connect to each other are also used in the Home Setting:
What is a Home Use Device?
A home use medical device is a medical device intended for users in any environment outside of a professional healthcare facility. This includes devices intended for use in both professional healthcare facilities and homes.
- A user is a patient (care recipient), caregiver, or family member that directly uses the device or provides assistance in using the device.
- A qualified healthcare professional is a licensed or non-licensed healthcare professional with proficient skill and experience with the use of the device so that they can aid or train care recipients and caregivers to use and maintain the device.
and other environments beyond professional health care facilities. Patients may have to use these devices for the duration of an illness, recuperation, long-term care, or throughout their lives.
A medical device is any apparatus, appliance, software, material, or other article—whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application—intended by the manufacturer to be used for human beings for the purpose of:
- Diagnosis, prevention, monitoring, treatment, or alleviation of disease;
- Diagnosis, monitoring, treatment, alleviation, or compensation for an injury or handicap;
- Investigation, replacement, or modification of the anatomy or of a physiological process;
- Control of conception; and which does not achieve its principal intended action in or on the human body by pharmacological, immunological, or metabolic means, but which may be assisted in its function by such means
Medical devices vary according to their intended use and indications. Examples range from simple devices such as: tongue depressors, medical thermometers, and disposable gloves to advanced devices such as computers which assist in the conduct of medical testing, implants, and prostheses. Items as intricate as housings for cochlear implants are manufactured through the deep drawn and shallow drawn manufacturing processes. The design of medical devices constitutes a major segment of the field of biomedical engineering.
The future of the global medical device market looks good with opportunities in public and private hospitals. The global medical device market is expected to reach an estimated $409.5 billion by 2023, and it is forecast to grow at a CAGR of 4.5% from 2018 to 2023. The major drivers for the growth of this market are healthcare expenditure, technological development, aging population, and chronic diseases.
Emerging trends which have a direct impact on the dynamics of the medical device industry include the changing medical technology landscape, software as a differentiator in medical devices, and design and manufacturing of patient portable and smaller devices.
Some of the medical device companies profiled in this report include Medtronic Public Limited Company, Johnson & Johnson, General Electric Company, Siemens AG, and Cardinal Health Inc. and others.
On the basis of comprehensive research, the researcher forecasts that the orthopedic device segment will show above average growth during the forecast period.
By function type, the global medical device market is segmented into diagnostic and monitoring, therapeutic, surgical, and others. The surgical segment is expected to remain the largest segment in the forecast.
North America is expected to remain the largest market during the forecast period mainly due to a large target patient pool coupled with a high adoption rates for advanced treatments in this region.

Artificial Heart Driver
- Advanced servo-controlled pneumatics and vacuum
- High-availability mission critical design
- Embedded computer platform technology
- Medical device electrical and electromagnetic compliant designs
- Redundant and diverse power source technology
- Industrial design elements for ease of use, portability within the hospital
- Pump “hotswap” capabilities for easily maneuvered ambulatory use
Balloon Catheter Inflation Device
- Low-cost disposable design
- MEMS-based technology to measure pressure
- Software and electronics developed to provide real-time balloon diameter data

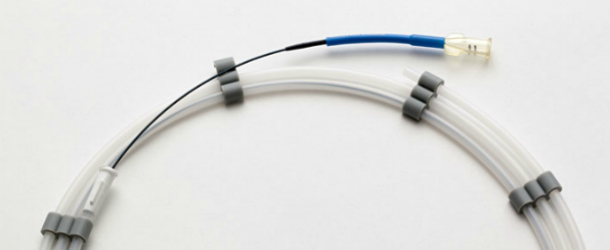
Catheters
- Catheter designs for single and multiple lumen applications
- Catheters designed for drug delivery, cardiology, endoscopy, neuromodulation and interventional vascular procedures
- Application-specific configuration and material selection
- Assembly capabilities that include bonding, ultrasonic welding, laser, lubrication and imprinting
Continuous Blood Glucose Monitor
- Continuous Physiological Monitoring
- Wireless Communication
- Ultra Low-Power
- Medical-Grade Software
- Intuitive User Interface
- Bi-Directional Wireless Communications
- Patient Data Retention
- Pictured above with skin abrator

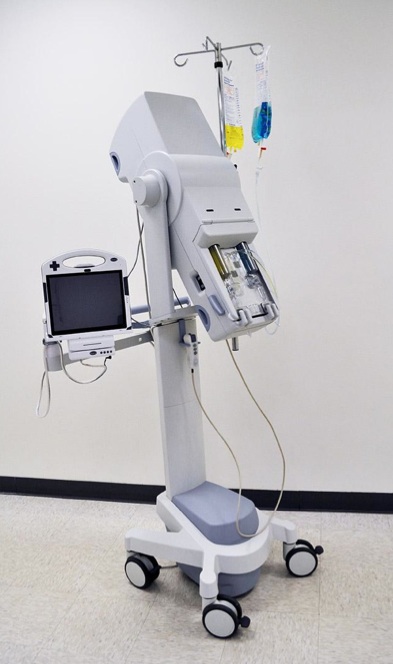
Contrast Media Power Injection Device
- Precise Fluid Control
- Power Injection
- Ergonomic User Interface
- Complex Systems Integration
- IEC 60601-24 and FDA Infusion Pump Guidance Compliance
Endoscope Cleaner / Disinfector
- “Concept-to-Customer” Development and Manufacturing
- Complex Electromechanical Fluid Handling
- Closed Loop Control Using Fluid Level, Pressure, Temperature and Ozone Feedback
- Medical-Grade Software


Implantable Arrhythmia Detector
- Hermetic packaging design and process
- Electrode and antenna encapsulation
Implantable Drug Infusion Pump
- “Concept-to-Customer” Development and Manufacturing
- Complex Electromechanical Fluid Handling
- Implantable Microelectronics
- Novel Implantable Materials
- Integrated Medical-Grade Software
- Programmable and Constant Flow Capability


Infusion Rate Monitor
- Measures infusion pressure into the brain
- Disposable pressure-monitoring hand piece syringe cradle
- Data acquisition electronics and software integration
- Clinician-friendly GUI for medical-grade tablet
Intelligent Chest Drainage Device
- Designed for Disposable High-Volume Manufacturing
- Mold Design for Optimum Ultrasonic Weld Process
- MEMS Pressure Measurement
- Battery Powered Electronics


Iontophoretic Drug Delivery Device
- Low-Cost, High-Volume Disposable Systems
- Designed Mold Tooling
- Ergonomic Design for Home Use
- Designed for Automated Assembly and Test
LVAD Pump Controller Console
- Mechanical and electrical engineering design improvements for functionality
- Simplified design features for ease of manufacture with an emphasis on cost point requirements for emerging market commercialization
- Supply chain and assembly process development and documentation


RF Ablation Control Console
- Industrial designed to meet customer requirements for ease of carrying
- Meets branding guidelines using color matching
- Conceptualized, designed and pilot manufactured RoHs compatible RF Ablation electronics
- New software architecture and GUI conceptualized, coded and implemented by UCT’s Affiliated Company
- Configured to be compatible with both legacy hand piece, as well as newly designed RF ablation hand piece
- Accommodates multi-language software GUI requirement
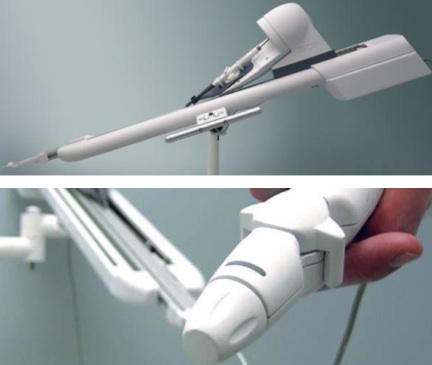
Remote Catheter Manipulation Device
- Paradigm Shifting Technology Platform
- Precise Motion Control
- Ergonomic User Interface
- Complex Systems Integration

Vascular Access Ports
- Catheter Material Selection
- Power Injectible and Non-Power Injectible
- Valved and Non-Valved
- Metal and Plastic Port Bodies
- Proprietary Interconnects
- Pressure & Flow Characteristics Optimized with CFD
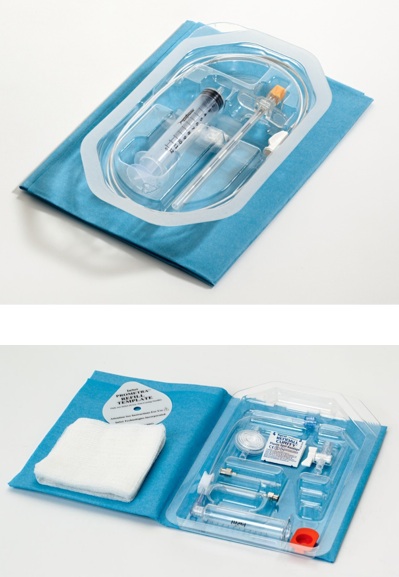
Sterile Disposable Kits
- Sterile Package Design
- Clean Room Assembly
- Packaging seal validation
- Shelf life accelerated aging testing

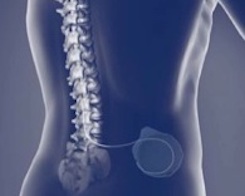
Wireless Programmer for Implantable Pump
- Digital signal processing
- High-speed, near-field wireless telemetry design
- Embedded software design and validation